The world’s oceans cover nearly two-thirds of the Earth's surface. Saltwater accounts for 97.5% of the world's water reserves, while freshwater makes up only 2.5%. Modern technologies enable the extraction of drinking water from saltwater at relatively low costs, yet untreated water is not considered drinkable. Instead of discussing technologies, let's explore what determines the concentration of salt in water.

Why is the ocean salty?
It all starts with rain. Water evaporates from the ocean’s surface, rising up to form clouds composed of tiny droplets of distilled water, which contain almost no dissolved particles. When these droplets grow larger and fall to the ground as rain, they come into contact with the atmosphere. One of the main components of air is carbon dioxide, which dissolves well in water, forming weak carbonic acid. As this solution falls to the ground, it slowly erodes various mineral rocks. The ions dissolve in the water of streams, which flow into rivers, and the rivers eventually empty into the ocean. Some of these impurities are absorbed by marine microorganisms or settle on the ocean floor as a result of chemical reactions, while others accumulate in the form of dissolved ions in the vast volumes of ocean water.
Another source of salts is the fluids released from hydrothermal vents on the ocean floor. Water seeps through cracks and fissures in the seabed and is heated by the magma within the Earth's core. This heating causes calcium, magnesium, and dissolved oxygen to be released from the water, while other minerals, such as iron, zinc, and copper, dissolve into it.
Less frequently, salts enter the water through underwater volcanic eruptions.
What salts are present in seawater?
If you were to taste seawater, it would be different from regular water in which 35 grams of salt were dissolved per liter, right?
Sodium chloride indeed makes up a significant portion of the salt content, but in reality, seawater contains many other mineral impurities. Below is a table showing the ions that make up 99.9% of the mass of sea salt.
| Ion | Concentration, ppm | Dry Salt Fraction, % |
|---|---|---|
| Chloride (Cl-) | 19345 | 55.03 |
| Sodium (Na+) | 10752 | 30.59 |
| Sulfates (SO4²-) | 2701 | 7.68 |
| Magnesium (Mg²+) | 1295 | 3.68 |
| Calcium (Ca²+) | 416 | 1.18 |
| Potassium (K+) | 390 | 1.11 |
| Bicarbonates (HCO3-) | 145 | 0.41 |
| Bromide (Br-) | 66 | 0.19 |
| Borate (BO3³-) | 27 | 0.08 |
| Strontium (Sr²+) | 13 | 0.04 |
| Fluoride (F-) | 1 | 0.003 |
Some of the elements are biogenic and are absorbed by algae and living microorganisms, while others simply remain dissolved in seawater. The salt concentration in seawater also affects the stability of fish and algae tissues. This is due to the osmotic effect, which causes water to move from an area with a higher concentration of salts to an area with a lower concentration.
How much salt is in a liter of seawater?
The average salinity of the world's oceans is 35‰ (or 35 PSU), which means that 35 grams of salt are dissolved in a liter of water. The average salinity is the arithmetic value obtained from multiple water samples taken from various locations around the globe.
How is salinity measured?
There are two methods for measuring the concentration of salt in water:
- Measuring how many grams of salt are in a liter of water by evaporating it (in this case, the units are expressed in per mille and denoted by "‰").
- Measuring the water's electrical conductivity (conductometry); in this case, salinity is measured in PSU (Practical Salinity Units).
It is clear that some water bodies contain more dissolved salts, while others contain less. This depends on several factors: precipitation, river inflows, and temperature. The ocean can be likened to a glass of water, as it is influenced by the same processes: dissolution, dilution, diffusion, etc. Of course, depending on the volume of water, these processes slow down and level out, but overall, the picture is very similar.
Precipitation
The amount of rain that falls around river basins flowing into the seas and oceans, as well as directly on the water's surface, plays perhaps the most important role. For example, the average salinity of the Atlantic Ocean is 35.4‰. In the subtropical zone, where there is very little rainfall, it is higher—37.25%. In the equatorial region, where it rains constantly, the salinity is lower—just 34‰.
The lowest salt content is often observed at the mouths of large rivers, where it can reach 18‰, due to the rapid mixing of large volumes of freshwater with seawater. In such cases, the salinity decreases significantly as you move away from the shore.
Evaporation
Strong solar activity, which leads to enhanced evaporation of water from the ocean surface, is one of the causes of increased salinity. This may explain why salinity in the ocean's deep layers is usually lower than at the surface.
Temperature
This factor also plays an important role. When the water temperature decreases, the solubility of most minerals drops as well, which is why cold currents are less salty than warm ones.
It is important to note that the solubility of the main component of seawater—sodium chloride (table salt)—depends little on temperature. Perhaps if another mineral were the basic component, fluctuations in the salt composition of water would make it less habitable for the various living organisms that can function in water thanks to osmotic processes.
Glaciers
Another important factor regulating salinity is the presence of ice. As the freezing point of saline solutions decreases with increasing concentration, during the formation of sea or ocean ice, a sort of concentration of pure water occurs, which freezes faster. Consequently, the remaining liquid water becomes saltier. When the ice melts, the water is diluted again and becomes less salty. This applies primarily to northern seas. In the case of the Arctic Ocean, which is the least salty, the process works in reverse.
The salinity levels of seas and oceans
We learned above that the average salinity of the world's oceans is 35‰, and what conditions affect it. Now let's take a closer look at where water is more or less salty.
The average salinity of the Atlantic Ocean is 35.4‰, with maximum salinity observed in the Mediterranean Sea (39‰), and in the open part of the ocean in the subtropics—up to 37.2‰. Minimum salinity is characteristic of the equatorial zone, where there are many rivers and rainfall.
The average salinity of the Indian Ocean is 34.8‰, with a maximum of 40-41‰ in the Persian Gulf and the Red Sea. In the Bay of Bengal, due to the freshwater influence of the Ganges, Brahmaputra, and other large rivers, salinity decreases (30-34‰).
The average salinity of the Pacific Ocean is about 34.5‰, with a maximum of 35.5-35.6‰. Salt content decreases in its eastern and northern parts.
The least salty is the Arctic Ocean. Its average salt content is just 32 g/L. The reasons for the low salinity of the Arctic Ocean are that due to low temperatures, little water evaporates from its surface, and it is constantly replenished by large river inflows and meltwater, which constantly reduce salt concentration through dilution. Interestingly, in this case, salinity increases with depth rather than decreases.
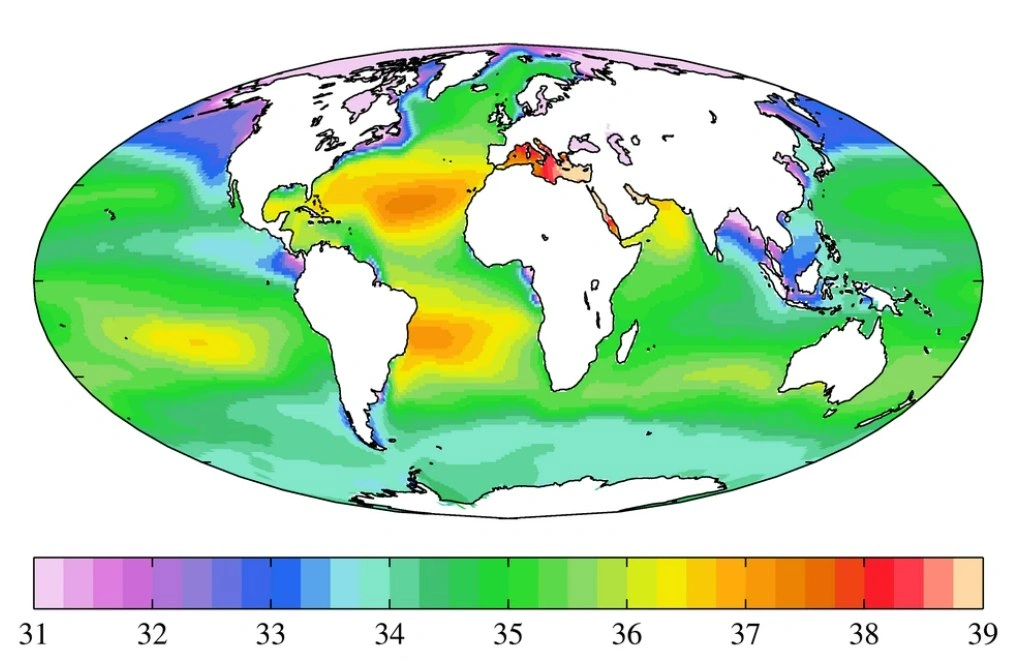
Salinity of the world's oceans
The salinity of seawater can vary greatly from oceanic water. If we recall our analogy with a glass, all the factors we mentioned above will affect the water composition more intensely.
Evaporation of the upper water layer due to intense solar radiation and low river inflow always indicates that salinity will be higher. For example, the Red Sea, which is surrounded by desert and has no freshwater sources, has the highest salinity (41 g/L). A similar situation is observed in the Mediterranean Sea: the colder the climate and the more rivers flow into it, the lower the salinity; the warmer, the higher.
The opposite situation is observed in the Baltic Sea, where the average salinity is just 7‰, but it varies greatly across different sections. For example, in the Danish Straits, which connect the Baltic Sea to the North Sea, it reaches 20‰, in the central part it is 6-8‰, and in the Gulf of Finland and the Gulf of Bothnia, where the highest river inflow occurs, it is only 2-3‰. It is important to note that in this sea, salinity also increases with depth.
Below is a table of sea salinities, which allows for comparing all the factors and assessing their impact on water salinity.
| Name | Salinity, ‰ |
|---|---|
| Baltic Sea | 7 |
| Sea of Azov | 11 |
| Black Sea | 18 |
| Marmara Sea | 26 |
| Adriatic Sea | 36 |
| Aegean Sea | 37 |
| Ligurian Sea | 38 |
| Mediterranean Sea | 39 |
| Red Sea | 41 |
Let’s briefly focus on the seas located near Ukraine: the Black Sea and the Sea of Azov.
The average salinity of the water in the Black Sea is 18 ‰, which is typical for the surface layer. The deeper layer is saturated with hydrogen sulfide, and its salinity reaches about 22 ‰. In this layer, there is practically no oxygen, and no organisms live there except for sulfur bacteria, which are responsible for producing the hydrogen sulfide.
As for the Sea of Azov, it has one of the lowest salinity levels—on average, 14 ‰, whereas 15 years ago it was only 9.5 ‰. The low salinity of the Sea of Azov is due to the large inflow of fresh water and the weak water exchange with the Black Sea. A unique feature of the Sea of Azov is that the predominant ionic components of its salinity are not chlorine and sodium ions, but calcium, sulfates, and carbonates.