Water has truly unique properties. Today, we will discuss its states of matter, the processes that occur within them, and discover at what temperature water freezes and evaporates. Water can exist in three states:
- Solid (ice)
- Liquid
- Gaseous (steam)
Why does water freeze and boil?
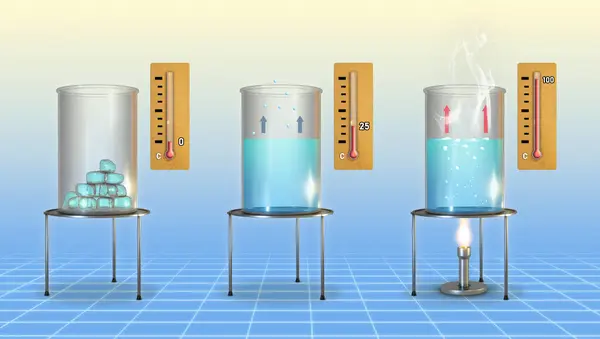
The structure of water is specific. In its liquid state, molecules are constantly moving and are only weakly bonded by hydrogen bonds. When heated, the molecules move more actively, colliding with each other, and eventually losing even weak bonds, turning into gas. This process is called boiling.
When the temperature drops, the bonds become stronger, and at the freezing point, the molecules form a hexagonal crystalline structure. You can observe this structure in snowflakes, which naturally arrange themselves into symmetrical shapes.
Reverse processes:
- Condensation - when water forms droplets as it cools down, like fogging on glass.
- Melting - when ice melts, such as in drinks or when snow turns into water.
Interestingly, hot water freezes faster than cold water.
Why does water expand when it freezes?

As mentioned earlier, hydrogen bonds tighten and form a structured arrangement when the temperature decreases. Ice crystals are built in such a way that the distance between molecules increases compared to their chaotic movement in liquid water. This results in ice having a lower density than water, meaning more molecules occupy less volume.
Freezing of purified water
When considering pure water as a chemical substance without any impurities, we can determine the freezing point of distilled water. However, natural or tap water always contains dissolved organic and inorganic substances that shift the equilibrium of phase transitions.
The freezing and melting point of distilled water at normal pressure is 0°C, while its boiling point is 100°C. These values were used as reference points for the Celsius temperature scale. In Kelvin, these temperatures are 273 K and 373 K, respectively.
Boiling point dependence on pressure
When pressure decreases, the boiling temperature of water drops, and when pressure increases, it rises. This explains why water boils faster at higher altitudes than at sea level. For example, at an altitude of 4,000 meters, water boils at 85°C (pressure - 0.6 atm), while at the summit of Mount Everest (8,448 meters), it boils at only 72°C.
The same applies to ice: under very high pressure, ice can melt even at room temperature. At a pressure of 0.006 atm, water cannot exist in a liquid state—it sublimates directly from ice to vapor.
| Pressure (atm) | Boiling Point (°C) |
|---|---|
| 0.01 | 7 |
| 0.05 | 29 |
| 0.1 | 45 |
| 0.6 | 85 |
| 0.987 (Normal Conditions) | 99.63 |
| 1 | 100 |
| 2 | 120 |
| 6 | 158 |
| 218 | 374.1 |
Freezing point of saltwater

Instead of using the term "salinity," it is more accurate to refer to the "total dissolved solids" (TDS) content. Any substances present in water shift the freezing point. For tap water, these shifts are in tenths or hundredths of a degree, which are not detectable by household thermometers. However, for seawater, the freezing point decreases noticeably, averaging around -1.8°C.
Each component in natural water contributes specific changes, and over 50 different substances can be present. For example, a 1.5% sodium chloride solution freezes at -0.9°C, while a 20% solution freezes at -16.6°C.
The freezing temperature of saltwater can be precisely calculated only if its exact composition is known.