If you put hot water in the freezer, you can observe the effect of its accelerated freezing. This phenomenon was previously mentioned by Aristotle, Francis Bacon, and René Descartes.
By placing two containers of hot and cold water in the freezer, you can see that the hot water will freeze faster. This fact is confirmed, for example, by the fact that in severe cold, open pipes with hot water freeze faster than cold pipes.
An experiment at home

To test the truth of this phenomenon, you can conduct a simple experiment at home.
- Take a liter of water and two separate ice cube trays.
- Pour about half of the water into a kettle and boil it.
- Fill the cold and heated water into the trays and put them in the freezer.
- Wait an hour and you will see which water turns into ice faster.
The Mpemba Paradox
In 1963, an African schoolboy noticed that a hot ice cream mixture in the freezer froze faster than a cold one. He did not get an answer to this question from his school physics teacher, but was able to ask physics professor Dennis Osborne. An experiment with water confirmed the effect. In this case, two 70 ml samples of water with a temperature of 25 and 90°C were placed in identical cups in the freezer of a household refrigerator on pieces of foam.
Osborne and Mpemba then conducted a series of experiments, the results of which were published in 1969 by the journal Physics Education.
The main points of the article are presented below.
Since cooling begins mainly from the upper surface of the liquid, the cooling rate depends on the temperature of this surface, not on the average temperature of the liquid, and convection processes maintain this temperature. As a result, the rate of heat loss for a system with a higher initial temperature will also be higher than for a more cooled system. The statement is controversial because water must pass through intermediate temperatures before freezing, but given the influence of the temperature gradient, the authors allowed that this statement could be omitted. After that, the phenomenon became actively discussed by researchers and was called the "Mpemba effect."
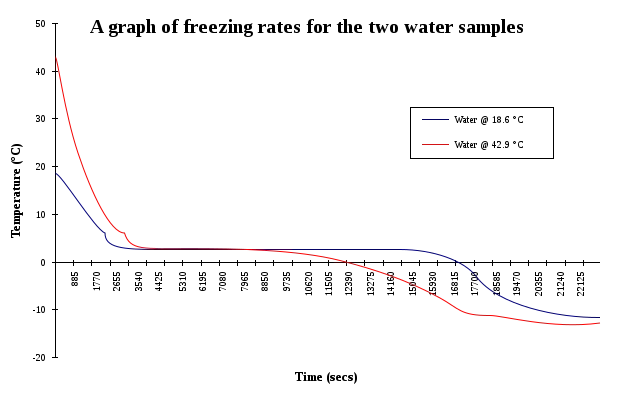
The figure shows the dependence of the freezing rate on the initial water temperature.
Explanation of the Mpemba effect
People have been looking for an answer to the question of why hot water freezes faster for half a century. Hundreds of scientific papers were published on the subject, but only 54 years later was a definitive answer obtained.
In 2013, the Royal Society of Chemistry of Great Britain promised to give a £1,000 prize to anyone who could explain the Mpemba effect. The best answer was an essay by Nikola Bregovic from the University of Zagreb in Croatia. He summarized the main theories studied earlier and described them.
In 2016, a group of scientists published research materials that denied the existence of this phenomenon. The explanation of the effect itself was based on research error.
It would seem that the scientific world should calm down, but not here, and in 2017, a joint study by a group of scientists from China and the United States explained the phenomenon by hydrogen bonds in the cluster structure of water.
The main theories
Water evaporation
Some scientists explained that heated water evaporates faster and, accordingly, either freezes in the air and forms an ice crust or is simply removed from the system. It is worth noting that in all the experiments where the mass of water was weighed before and after freezing, the maximum mass loss was no more than 3%. Such an insignificant change in mass obviously cannot cause a significant acceleration of freezing. Another difficulty with this experiment was that it was almost impossible to prove this point, since sealing the container with frozen water would change not only evaporation but also the movement of heat flows.
Dissolved gases
The solubility of gases in water decreases with increasing temperature. Based on this, some researchers assumed that the rapid freezing of water is related to this fact. Thomas's research showed that the difference in freezing temperatures deviates slightly from zero, and Auerbach proved that the concentration of gases in water does not affect supercooling.
Conversion is enhanced by a heat gradient
Let's understand what convection and heat gradient are. When a container of water is placed in a freezer, the liquid on the surface and near the walls of the container comes into contact with the cold environment and cools faster. At the same time, the temperature inside the sample is maintained, resulting in a temperature difference or temperature gradient in the container. It causes heat transfer, and the stronger the gradient, the better the convection. Accordingly, the higher the temperature difference, the more active the heat transfer and cooling will be.
Hydrogen bonds
In 2017, the final answer was given to the question of why hot water freezes faster than cold water. The reason is the properties of hydrogen bonds. The key argument of the researchers is that the number of strong hydrogen bonds increases with increasing temperature, and the existence of small strongly bonded clusters, in turn, contributes to the formation of regular hexagonal ice when warm water cools rapidly. In addition, the opposite effect of rapid heating of supercooled water has been proven.
We have already written about the formation of ice and the location of the water molecule in its structure.
By the way, the research is ongoing :)
Resources:
- Tao, Yunwen; Zou, Wenli; Jia, Junteng; Li, Wei; Cremer, Dieter (2016). Different Ways of Hydrogen Bonding in Water - Why Does Warm Water Freeze Faster than Cold Water?. ACS Publications. Collection. https://doi.org/10.1021/acs.jctc.6b00735
- N. Bregović, Mpemba effect from a viewpoint of an experimental physical chemist. http://www.rsc.org/images/nikola-bregovic-entry_tcm18-225169.pdf 2012.
- Different Ways of Hydrogen Bonding in Water - Why Does Warm Water Freeze Faster than Cold Water? Yunwen Tao, Wenli Zou, Junteng Jia, Wei Li, and Dieter Cremer. Journal of Chemical Theory and Computation 2017 13 (1), 55-76. DOI: 10.1021/acs.jctc.6b00735.