Water is the true source of life on earth, participating in countless biological processes and being an integral component of them. This is possible thanks to its unique properties such as:
In our blog, we often refer to certain properties of water, but rarely delve into the physics and chemistry of it. Today, we will try to break it out for you in a simple language why water is not only the "source of life on earth", but also a truly special chemical substance, highlighting its remarkable physical and chemical properties.
Physical Properties of Water
Pure water has no taste or odor and is in a liquid state at normal temperature (20°C).
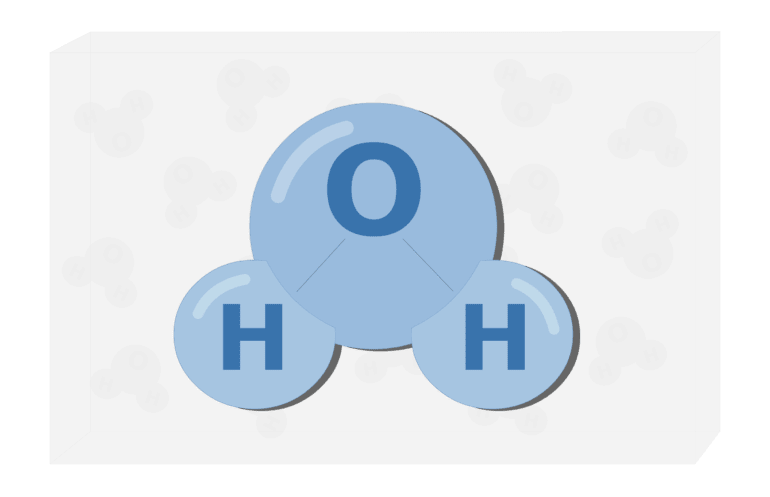
"Water" is a trivial name; the chemical compound is called hydrogen oxide. From the name, we can understand that it contains hydrogen and oxygen ions that are connected by a so-called covalent bond.
The hydrogen atom has a valence (ability to form bonds) of 1, and the oxygen atom has a valence of 2. This is why the formula for water is H2O. Also, each water molecule can form up to four hydrogen bonds (two hydrogen and two oxygen). All anomalies of the physical properties of water are related to these bonds - water has a relatively high boiling point (100°C). If there were no hydrogen bonds, water would boil at a temperature of -80°C and freeze at -100°C. This structure allows us to see water in three states (solid, liquid, gas) in the natural environment. Here, we just briefly described the physical properties of water, if you want to find out more about it, you are welcome to read our previous article where we’ve talked more about how water boils and freezes, as well as the differences between heavy water.
Chemical Properties of Water
Let's delve deeper into the chemical properties of water and how they relate to the indicators of water on Earth.
To understand processes in the environment that involve water as a component of reactions, it is important to be familiar with the main chemical properties of H2O. These properties can be summarized in a short list.
Interaction of water with simple substances
The chemical characteristics of water become evident in its reaction with alkaline and alkaline-earth metals, which can be quite vigorous, producing heat and occasionally even light. For instance, sodium, potassium, and calcium are able to move and even "jump" on the surface of water.
2Na + 2H2O = H2 + 2NaOH
Less active metals react either upon heating or not at all, for example, iron:
3Fe + 4H2O = 4H2+ Fe3O4 (only upon heating)
These reactions do not occur naturally, but the corrosion reaction that occurs when air is added to water is very common, showcasing another chemical property of water.
4Fe + 3O2 + 6H2O ➝ 4Fe(OH)3.
This equation describes the formation of rust on iron surfaces. Similar processes can also occur with copper, zinc, and their alloys. Reactions with non-metals occur exclusively upon heating or other types of influence.

Reactions with non-oxides
Water in nature often comes into contact with carbon dioxide, as well as sulfur and nitrogen oxides, which are components of exhaust gases, through this mechanism:
SO2 + H2O = H2SO4.
As a result of these processes, acid rain is formed, further illustrating the chemical characteristics of water.

Photosynthesis
Photosynthesis is a remarkable reaction that enables plants to convert carbon dioxide and water into nutrients, including starch and glucose, using sunlight as an energy source.
The chemical equation for photosynthesis is:
6nCO2 + 5nH2O → (C6H10O5)n + 6nO2
This equation summarizes the process by which plants use water to create carbohydrates and release oxygen. As such, photosynthesis is a critical process that sustains life on Earth by generating oxygen and providing the foundation for many food chains in ecosystems.

Water as an ideal solvent
Water is a versatile solvent that facilitates many invisible processes, which are often the most fascinating ones. However, it is rare to find water in its purest form in nature, as it typically contains inorganic salts, gases, and a diverse range of organic substances. The natural hardness of water is a result of its movement through rocks, which allows it to become saturated with minerals. Depending on the composition of the rocks, their solubility, and the temperature of the environment, certain concentrations of their ions can dissolve in water. Carbonates, sulfates, nitrates of calcium, magnesium, sodium, potassium, and other cations are commonly found in such rocks. Minerals such as gypsum (CaSO4), dolomite (CaCO3 • MgCO3), and limestone (CaCO3) are likely the primary contributors to water hardness.

Dissolved iron and manganese are typically present in natural borehole waters, as soluble iron salts tend to be found in areas lacking air. Their sources are often magnetic, brown, red iron ores, magnesite, and similar materials. Hydrogen sulfide is also present in groundwater, where it is produced through chemical processes involving organic substances. In the air, it readily oxidizes to elemental sulfur and precipitates, often without being visible to the human eye.
Natural surface waters contain dissolved oxygen and nitrogen-containing components that result from the vital activity of microorganisms, such as ammonia, nitrites, and nitrates, which can easily convert into one another. Proteins and amino acids can also be found in water bodies. Anthropogenic activities introduce the most toxic pollutants into the water, such as heavy metal salts, industrial organic products, and nitrates and phosphates from fertilizers.

Various reactions occur constantly in water, including exchange processes that cause scale consisting of calcium carbonate to precipitate. Additionally, oxidizing-reducing reactions can result in borehole water acquiring rusty iron sludge or sediment.
In summary, the chemical and physical properties of water make it an exceptional substance with diverse applications. As an ideal solvent, water's ability to dissolve various substances and exhibit unique physical traits like high surface tension and thermal conductivity contributes to its widespread utility in biological and industrial settings. By describing the physical and chemical properties of water, we gain insight into its pivotal role in sustaining life and driving essential natural processes.
Faqs
-
How do water's chemical properties affect its role in biological systems?
Water's chemical properties are vital for life's processes. Its ability to act as a solvent allows for the transport of nutrients, minerals, and waste products within living organisms. Water's capacity to participate in chemical reactions as an acid or base is essential for maintaining pH balance in biological systems. Additionally, water's cohesion and surface tension enable capillary action, facilitating the movement of water through plants and the blood vessels of animals. Its electrolytic properties are also fundamental for many biochemical reactions that occur within cells.
-
What is the importance of water's physical properties?
The physical properties of water play a crucial role in supporting life and shaping the Earth's environment. Water's high boiling and freezing points allow it to exist as a liquid over a wide range of temperatures, creating stable habitats for aquatic organisms. Its ability to dissolve and transport various substances enables nutrient absorption in plants and animals. The expansion of water when it freezes helps protect aquatic life in cold environments. Additionally, water's high specific heat capacity regulates temperature on Earth, influencing climate patterns and weather systems.
-
What are the physical properties of water?
Water possesses several important physical properties. It is a colorless, odorless, and tasteless liquid at room temperature. Its boiling point is 100 degrees Celsius (212 degrees Fahrenheit), and its freezing point is 0 degrees Celsius (32 degrees Fahrenheit). Water is unique in that it expands when it freezes, causing ice to float on its liquid form. It also has a high surface tension and can absorb a significant amount of heat before its temperature rises, making it an effective coolant.
-
What are the chemical properties of water?
Water exhibits various chemical properties due to its molecular structure. It is composed of two hydrogen atoms bonded to one oxygen atom, forming a molecule with the chemical formula H2O. Water is a versatile solvent, capable of dissolving many different substances, which is vital for biological processes. It also acts as both an acid and a base, meaning it can donate and accept protons. Additionally, water undergoes electrolysis, splitting into hydrogen and oxygen gases when an electric current is passed through it.